Abstract
The pharmaceutical and biologics industries face growing debate over whether to reduce established endotoxin limits to improve patient safety, particularly in parenteral therapies. This white paper critically evaluates proposals to halve endotoxin limits based on the hypothetical risk of “endotoxin stacking” and instead argues for a science-driven approach centered on environmental control and rigorous testing. Drawing on regulatory standards such as USP <85> and using Aquabiliti’s endotoxin monitoring practices as a case study, we demonstrate that effective mitigation through manufacturing controls provides a more practical and evidence-based safeguard than reducing allowable limits without supporting data. Our analysis supports maintaining current regulatory limits while emphasizing precision in contamination prevention.
Background
There have been debates over whether there should be a reduction in the established endotoxin limits for drugs and biologics to improve patient safety. In some instances, there have been proposals to reduce the endotoxin limits by half to account for the potential of each component of a therapy being at the high end of its endotoxin limit. The concern driving this push for reduction is that endotoxin contamination can lead to a febrile response in patients undergoing parenteral therapies. While proven documentation of this “endotoxin stacking” does not exist in the public domain, many groups are advocating for a re-examination of how to establish endotoxin limits.
Endotoxins are the structural components found on the outer membrane of gram-negative bacteria. The current method of establishing endotoxin limits is based on a calculation considering the threshold pyrogenic dose and the patients’ weight. Introduction of the LAL test in the 1970s–80s serves as the primary alternative to the rabbit pyrogen test (RPT) and is a more sensitive test that can detect lower levels of endotoxin, ensuring better safety for patients. This is especially indicative for environmental endotoxins, which can come from the air, water, and surfaces of the manufacturing environment.
Endotoxin stacking in the manufacturing process refers to the cumulative effect of endotoxins introduced at various stages of production. These endotoxins can contaminate pharmaceutical products during the manufacturing process. This endotoxin contamination can occur through raw materials, water, and equipment, such as tanks and their associated mixers, and even during handling and storage.
Proposed Solution
Rather than reducing endotoxin limits as some have proposed, the key to controlling endotoxin is to mitigate the risk of environmental endotoxins by implementing strict controls in manufacturing facilities. These controls would include a strong cleaning program, regular environmental monitoring, and using endotoxin-free materials and equipment whenever possible. Implementing these controls in conjunction with utilizing the calculation derived per USP <85> for establishing endotoxin limits creates more stability than reducing the endotoxin limit by 50% to account for a potential endotoxin stacking not seen in the public.
Aquabiliti utilizes the kinetic chromogenic assay as described in USP <85> to measure the color intensity related to the endotoxin concentration in the test samples of our bulk-filled water. Acceptable endotoxin limits are set by USP <85> for injectable drugs, medical devices, and other products. For WFI, Sterile WFI, and Sterile Water for Irrigation, the allowable limit is 0.25 EU/mL (endotoxin units/milliliter).
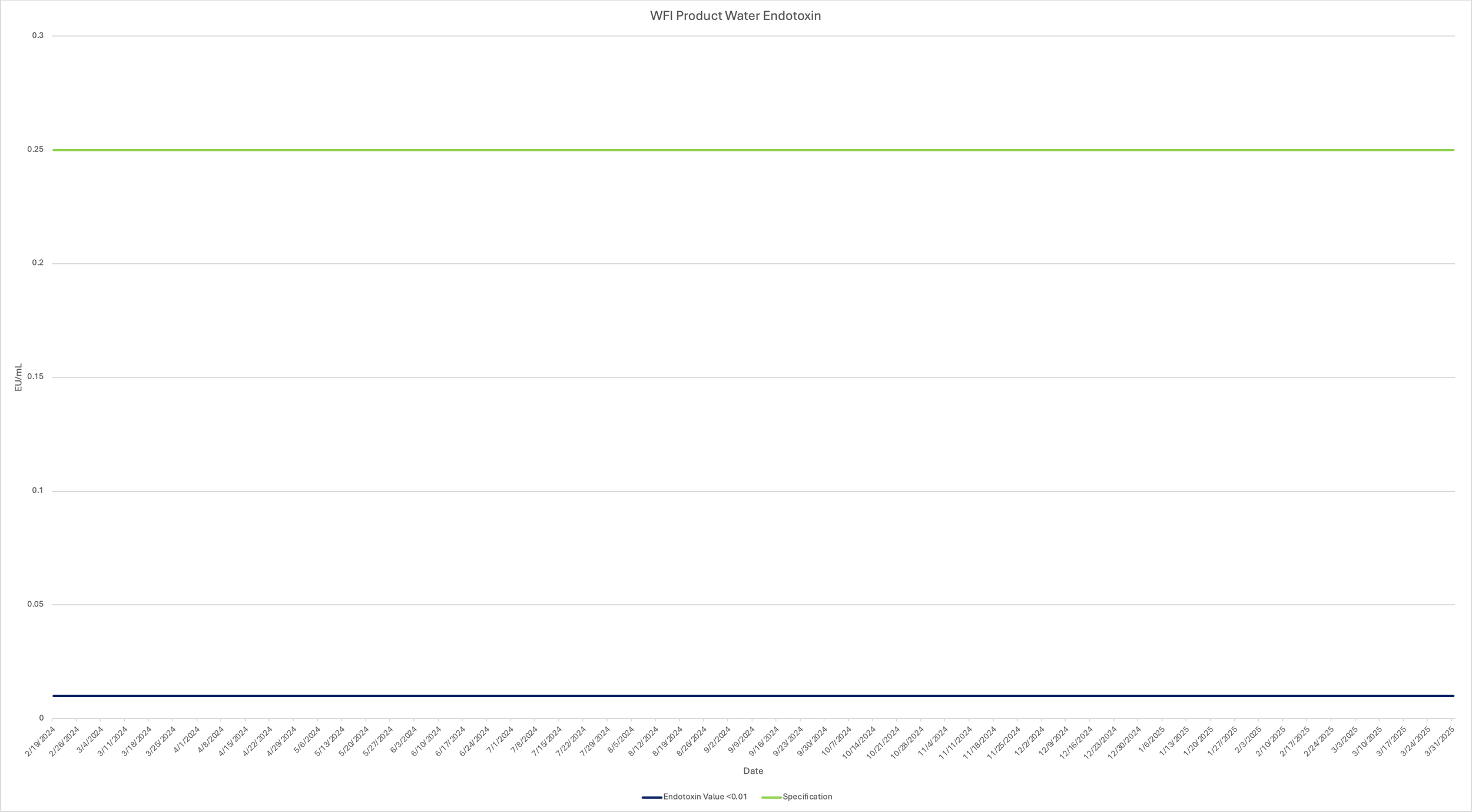
Aquabiliti understands the importance of endotoxin as it relates to parenteral therapies and monitors our WFI system weekly for endotoxin in addition to ensuring the finished product has a low endotoxin result as required by the customer. To demonstrate the stability of our water regarding endotoxin levels, review the chart for the rolling calendar year for endotoxin results for our WFI system. (Click image to enlarge.)
Aquabiliti utilizes a test system primarily designed for aqueous solutions near a neutral pH, so it is ideal for evaluating WFI. As shown in the chart legend, our samples receive a value of <0.01 EU/mL. This means that the endotoxin level in the sample is below the detection limit of the cartridge being used, which is 0.01 EU/mL, indicating that the endotoxin concentration in the water we fill is too low to be accurately quantified by the recommended test cartridge. This is useful for confirming that the sample has exceptionally low endotoxin levels, which is a requirement when utilizing WFI for parenteral therapies.
At Aquabiliti, we ensure that the bulk water our customers receive as a critical raw material does not contribute to the downstream introduction of endotoxin in the manufacturing process.
Summary
In response to calls for more stringent endotoxin limits in drugs and biologics, this white paper presents an alternative perspective rooted in scientific validation and real-world manufacturing practices. While concerns about endotoxin stacking have led to suggestions to halve current limits, public data do not substantiate such risks.
Instead of arbitrary reductions, Aquabiliti advocates for a proactive strategy focused on:
-
Implementing strong environmental monitoring and sanitation protocols.
-
Using validated endotoxin-free materials and equipment.
-
Applying regulatory standards, specifically USP <85>, to calculate safe thresholds.
Aquabiliti’s case study illustrates the successful application of kinetic chromogenic assays to consistently achieve <0.01 EU/mL endotoxin levels in Water for Injection, far surpassing industry standards. This underscores that patient safety is best served through robust process controls, not blanket regulatory tightening.
This white paper encourages industry stakeholders and regulators to reconsider risk-mitigation strategies and focus on measurable, effective contamination control over theoretical risk reduction.
Reference